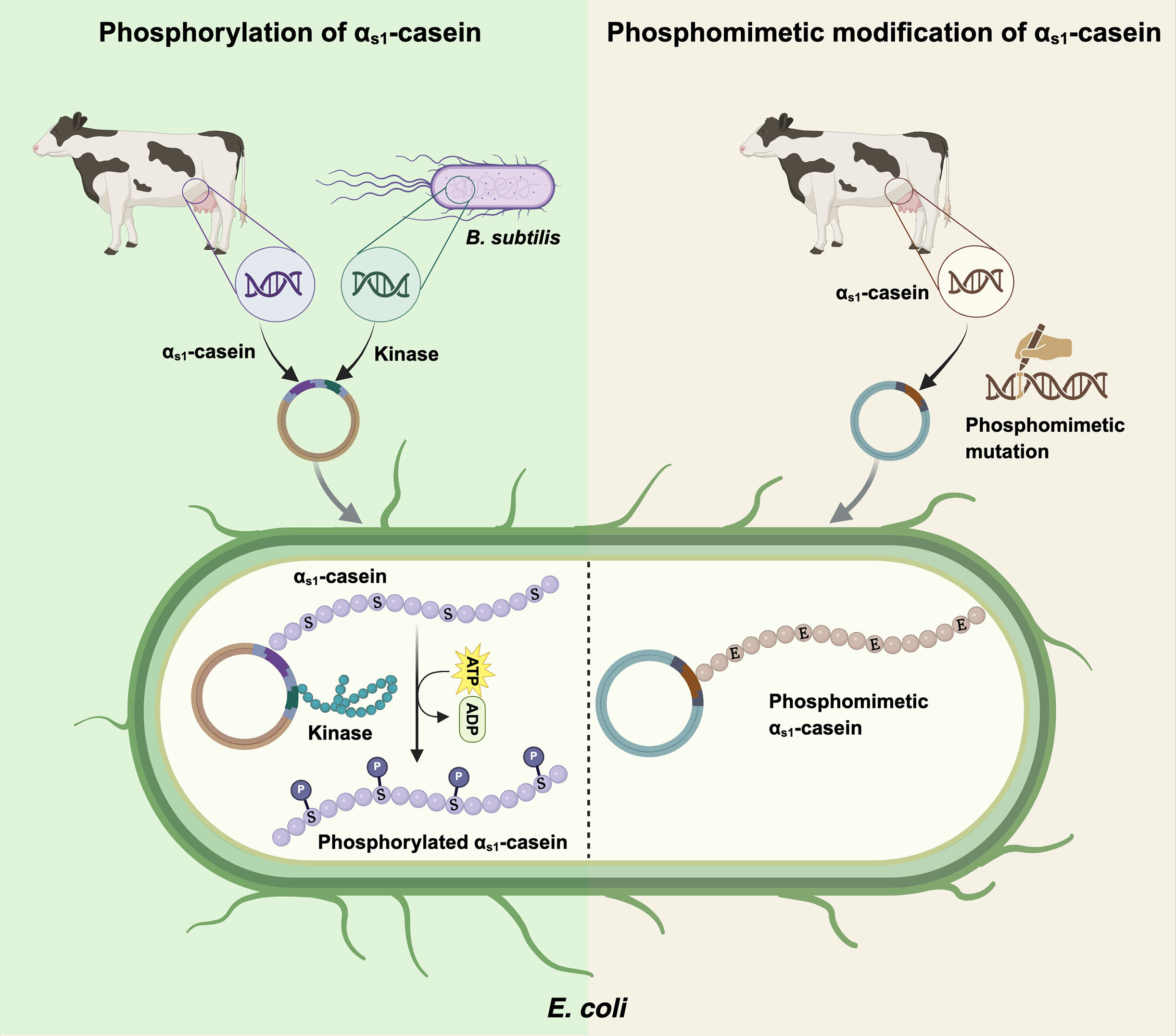
The study demonstrates two strategies, phosphorylation of αs1-casein using Bacillus subtilis kinases and phosphomimetic substitution of αs1-casein. Credit: Trends in Biotechnology (2025). DOI: https://doi.org/10.1016/j.tibtech.2025.05.015
Breakthrough Study Offers Animal-Free Path to Cheese and Yogurt
In a major step toward sustainable dairy, scientists have successfully engineered Escherichia coli bacteria to produce functional milk proteins—without a single cow in sight. This development could power a new generation of plant-based cheeses and yogurts that match traditional dairy at the molecular level.
Why Casein Matters
Casein is the main protein in milk that gives cheese its stretch, yogurt its creaminess, and delivers essential amino acids to the body. It also forms micelles, microscopic structures that help bind and deliver calcium. Traditionally, casein comes from cow’s milk, but that production comes at a high environmental and ethical cost.
The Challenge of Phosphorylation
One of the barriers to making functional casein outside animals is a biological process called phosphorylation. This chemical modification—where phosphate groups attach to specific amino acids—is what gives casein its calcium-binding power and its ability to form micelles.
Previous attempts to create recombinant casein using microbes missed this critical step. Without phosphorylation, the proteins lack both functionality and nutritional equivalence to real dairy.
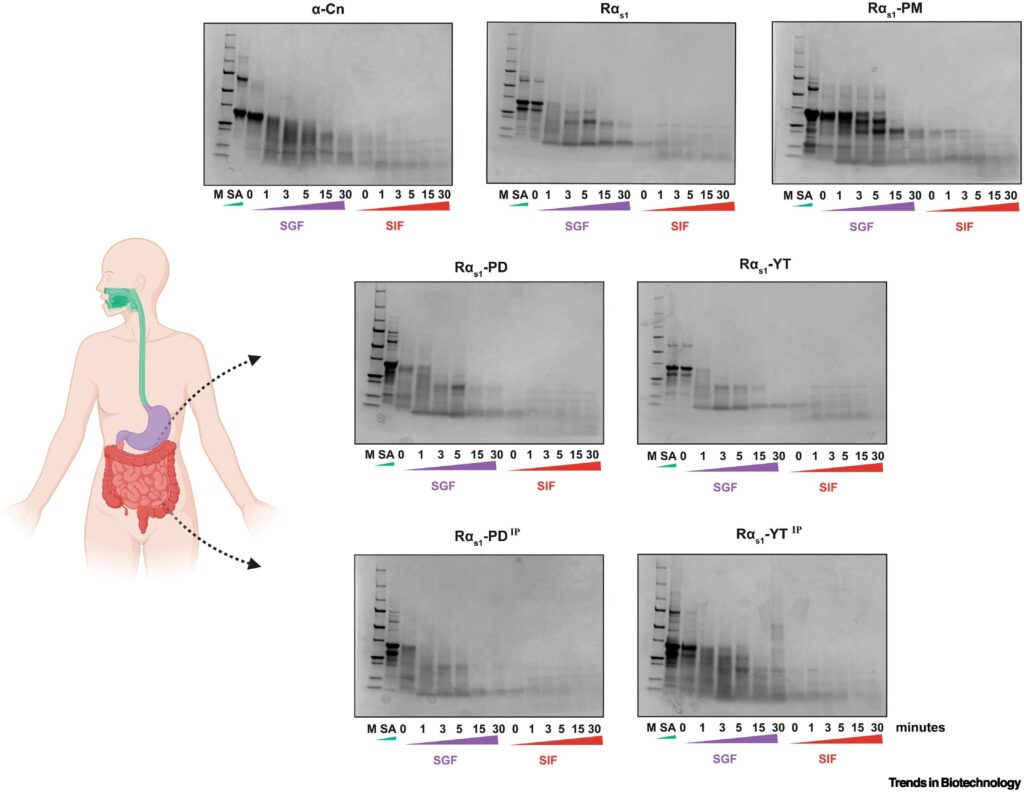
In vitro simulated gastrointestinal digestion of caseins. Credit: Trends in Biotechnology (2025). DOI: 10.1016/j.tibtech.2025.05.015
Two Solutions from the Lab
To overcome the phosphorylation problem, the research team developed two methods:
- Kinase Co-expression: They engineered E. coli to produce not just αs1-casein, but also three specific Bacillus subtilis kinases—enzymes that add phosphate groups to proteins during synthesis.
- Phosphomimetic Substitution: In this approach, they substituted certain serine residues (normally phosphorylated in cow’s milk) with aspartic acid, a chemically similar amino acid that mimics the effect of phosphorylation.
Functional Tests Show Strong Results
To validate the functionality of the bacterial casein, researchers conducted:
- Calcium-binding assays to test the protein’s nutritional role
- Structural analyses to compare shape and integrity with native casein
- Simulated digestion to see how well the proteins break down in the human gut
The results? Both versions—phosphorylated and phosphomimetic—showed strong calcium-binding ability, stable micelle formation, and digestibility on par with cow-derived casein.
Implications for the Future of Dairy
This research opens up a realistic path toward animal-free dairy products that don’t just imitate milk—they recreate it. Because these proteins are molecularly similar to what you’d find in cow’s milk, they could be used in existing dairy manufacturing setups with minimal changes.
The team noted that kinase-based phosphorylation may offer the closest match to native casein, while the phosphomimetic method could simplify scale-up and reduce production costs.
What’s Next?
Further research is needed to refine both approaches and confirm large-scale viability. Still, this is a major step toward cruelty-free, environmentally friendly milk protein alternatives that don’t compromise on nutrition or performance.
Reference
Study: Suvasini Balasubramanian et al. Production of phosphorylated and functional αs1-casein in Escherichia coli, Trends in Biotechnology (2025). DOI: 10.1016/j.tibtech.2025.05.015