A Crystal Clear Idea
In a dazzling display of scientific creativity, researchers in Taiwan have discovered that the performance of a clean energy catalyst can depend not just on what it’s made of—but also how it’s shaped.
A team from National Taiwan University, National Tsing Hua University, and National Yang Ming Chiao Tung University has shown that by precisely controlling the shape of cuprous oxide (Cu₂O) crystals, they can enhance how well these materials power fuel cells—a key green technology that could replace fossil fuels.
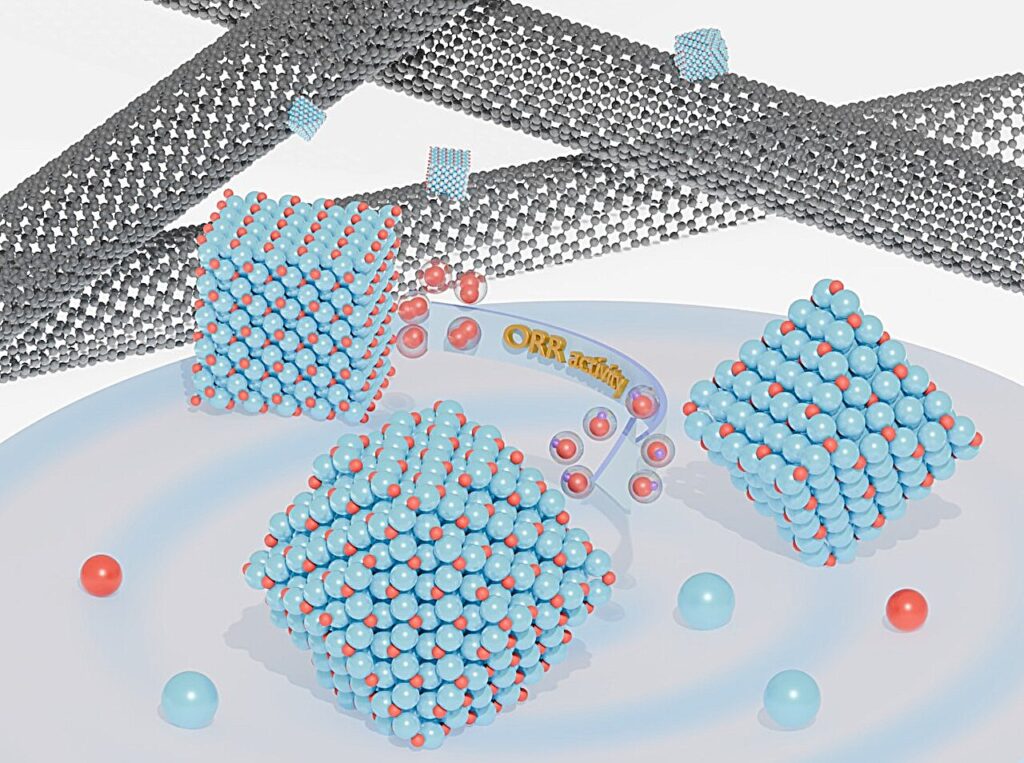
Schematic oxygen reduction reaction activity comparison of Cu2O polyhedral catalysts on CNT matrix. Credit: Prof. Jyh-Pin Chou
The results were published in the prestigious Journal of Materials Chemistry A.
Why Fuel Cells Need Better Catalysts
Fuel cells generate electricity through chemical reactions, often relying on expensive materials like platinum to speed up the oxygen reduction reaction (ORR)—a crucial step in the process. But platinum is rare and costly, making it an obstacle for scaling clean energy.
That’s where Cu₂O comes in. It’s abundant, low-cost, and—thanks to this new research—surprisingly versatile. The twist? Its effectiveness depends on its geometry.
The Magic of Shape: Cube, Octahedron, or Rhombic?
The researchers created Cu₂O crystals in three distinct shapes:
- 🟦 Cubes, showing {100} facets
- ⬢ Octahedra, exposing {111} facets
- ♦️ Rhombic dodecahedra, revealing {110} facets
Each shape was combined with carbon nanotubes to make them more conductive. But here’s where things get fascinating—each shape changed how the catalyst interacted with oxygen.
🔍 Using quantum simulations and microscopic imaging, the team found that the {110} facet (from the rhombic dodecahedron) had the most favorable interaction with oxygen, making it the most efficient shape for catalyzing the ORR.
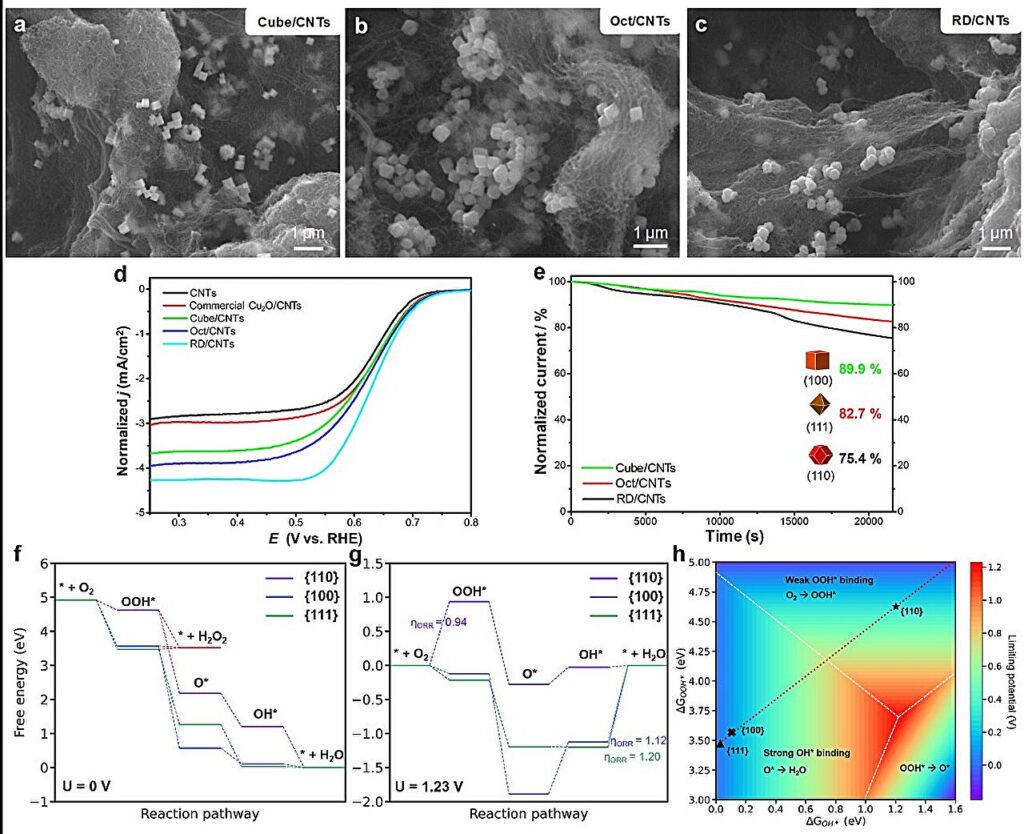
SEM images of Cu2O (a) cubes, (b) octahedra, and (c) rhombic dodecahedra mixed with carbon nanotubes. (d) Normalized LSV polarization curves for the ORR using different catalysts. (e) Chronoamperometric curves for the Cu2O/CNTs catalysts measured at 0.65 V vs. RHE in an O2-saturated 0.1 M KOH solution. Free energy diagram for oxygen reduction on Cu2O {100}, {110}, and {111} surfaces with (f) U = 0 V and (g) U = 1.23 V. (h) 2D volcano plot for the oxygen reduction reaction. The theoretical limiting potential represents the ORR activity. Credit: Prof. Jyh-Pin Chou
“We found that oxygen molecules adsorb and react differently depending on the crystal’s surface,” explained lead researcher Prof. Jyh-Pin Chou. “This gives us a powerful tool to engineer better catalysts from the ground up.”
Power vs. Durability: A Trade-Off
While the rhombic dodecahedra were star performers in terms of power output, they degraded faster due to self-oxidation. On the other hand, cube-shaped crystals were more stable over time, making them ideal for long-term use, even if their activity was slightly lower.
It’s a classic engineering puzzle: efficiency vs. endurance. And now, scientists can tailor their materials based on what the application needs most.
What This Means for Our Clean Energy Future
This study opens an exciting path forward—using crystal engineering to design affordable, high-performance catalysts that don’t rely on scarce metals. With further development, these Cu₂O structures could make fuel cells cheaper and more efficient, bringing us one step closer to a cleaner, greener energy future.
And it all starts with something as simple—and as extraordinary—as the shape of a crystal.
🌍 Could tiny crystals with precise angles be the key to powering our world sustainably?
Stay curious and visit www.dailyscitech.com for daily sci-tech updates.The next big energy breakthrough might already be growing in a lab.