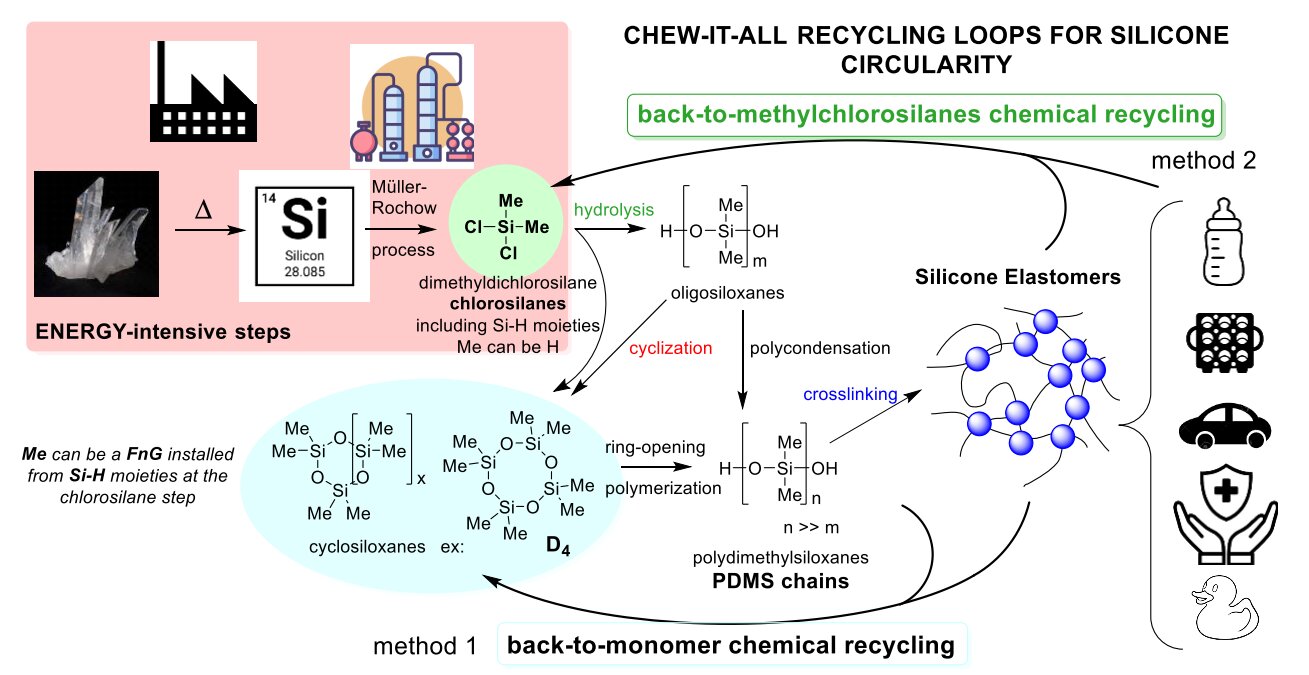
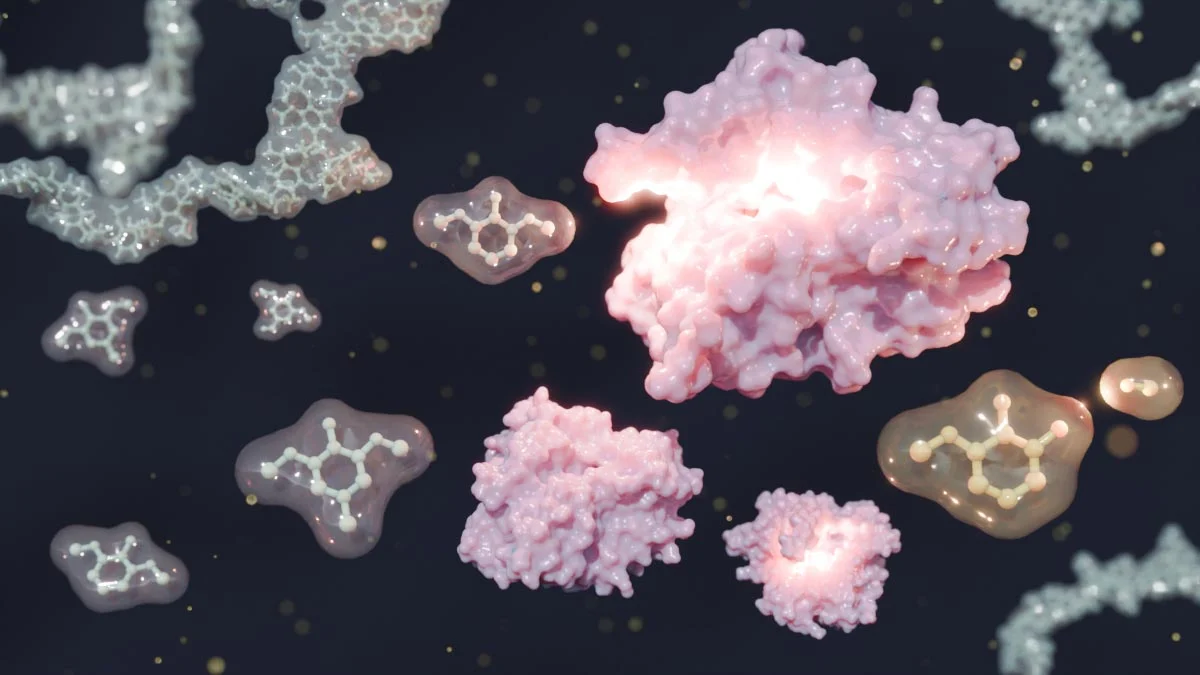
General strategies for the chemical recycling of silicone polymers. Credit: Nam Đức Vũ et al
Silicone’s Superpower… and Its Problem
Silicone is a modern marvel. It’s heat-resistant, durable, flexible, and found in everything from cookware to cosmetics, electronics, medical devices, and solar panels. But that long-lasting strength comes with a major drawback—silicone is notoriously hard to recycle.
Traditional methods either burn it (releasing harmful emissions) or dump it in landfills, where it can persist for hundreds of years. Until now, there’s been no clean or scalable way to break silicone down and reuse it without degrading the material’s quality.
A Game-Changing Chemical Discovery
Scientists from the French National Centre for Scientific Research (CNRS) have found a breakthrough solution. Led by Christophe Thomas at Chimie ParisTech, the team developed a chemical recycling technique that disassembles silicone into its purest parts—chlorosilanes—which are the core ingredients used to make brand-new silicone.
Using a gallium-based catalyst paired with boron trichloride, the method gently breaks the strong Si–O (silicon-oxygen) bonds found in silicone polymers. Instead of producing low-grade byproducts, the result is a high-yield batch of chlorosilanes, which can be re-polymerized into new, top-quality silicone.
This approach effectively reverses the silicone manufacturing process, making it fully circular.
Why This Matters for the Planet
The traditional production of silicone is energy-intensive, requiring raw quartz to be processed at extreme temperatures. This contributes heavily to greenhouse gas emissions and resource depletion.
By recovering chlorosilanes from waste silicone, this new method:
- Reduces dependence on raw silica and mining
- Slashes energy consumption
- Cuts down CO₂ emissions
- Helps industries transition toward a circular economy
The potential environmental savings are enormous—especially if this method scales up to industrial levels.
Not Just a Lab Experiment
What makes this research especially promising is that the process works under mild conditions, doesn’t require rare metals, and produces easily isolatable products. It’s efficient and potentially inexpensive to implement at larger scales.
Already, the team is collaborating with industry partners to explore commercial applications in sectors like:
- Medical silicone recycling
- Electronics and tech waste
- Cosmetic packaging recovery
- Silicone-based sealants and construction materials
This could reshape how manufacturers design and handle silicone-containing products from the start.
Get more information: Nam Đức Vũ et al, Gallium-catalyzed recycling of silicone waste with boron trichloride to yield chlorosilanes, Science (2025). DOI: 10.1126/science.adv0919. www.science.org/doi/10.1126/science.adv0919
Could Other Polymers Be Next?
This breakthrough opens the door to similar chemical recycling strategies for other high-performance but hard-to-recycle materials like fluoropolymers or resins. If scientists can identify suitable catalysts and conditions, even more synthetic materials could become infinitely recyclable.
It’s a thrilling possibility: what if zero-waste design wasn’t just a dream, but the future of materials science?
One Step Closer to a Circular Future
By turning old silicone into pristine raw materials without environmental harm, this discovery could help us rethink not just recycling—but manufacturing itself. Instead of a linear “make-use-dispose” model, we move closer to a closed-loop system where nothing is truly wasted.
Are we on the brink of a materials revolution?
Let us know what you think—how else could chemical recycling transform the products we use every day?