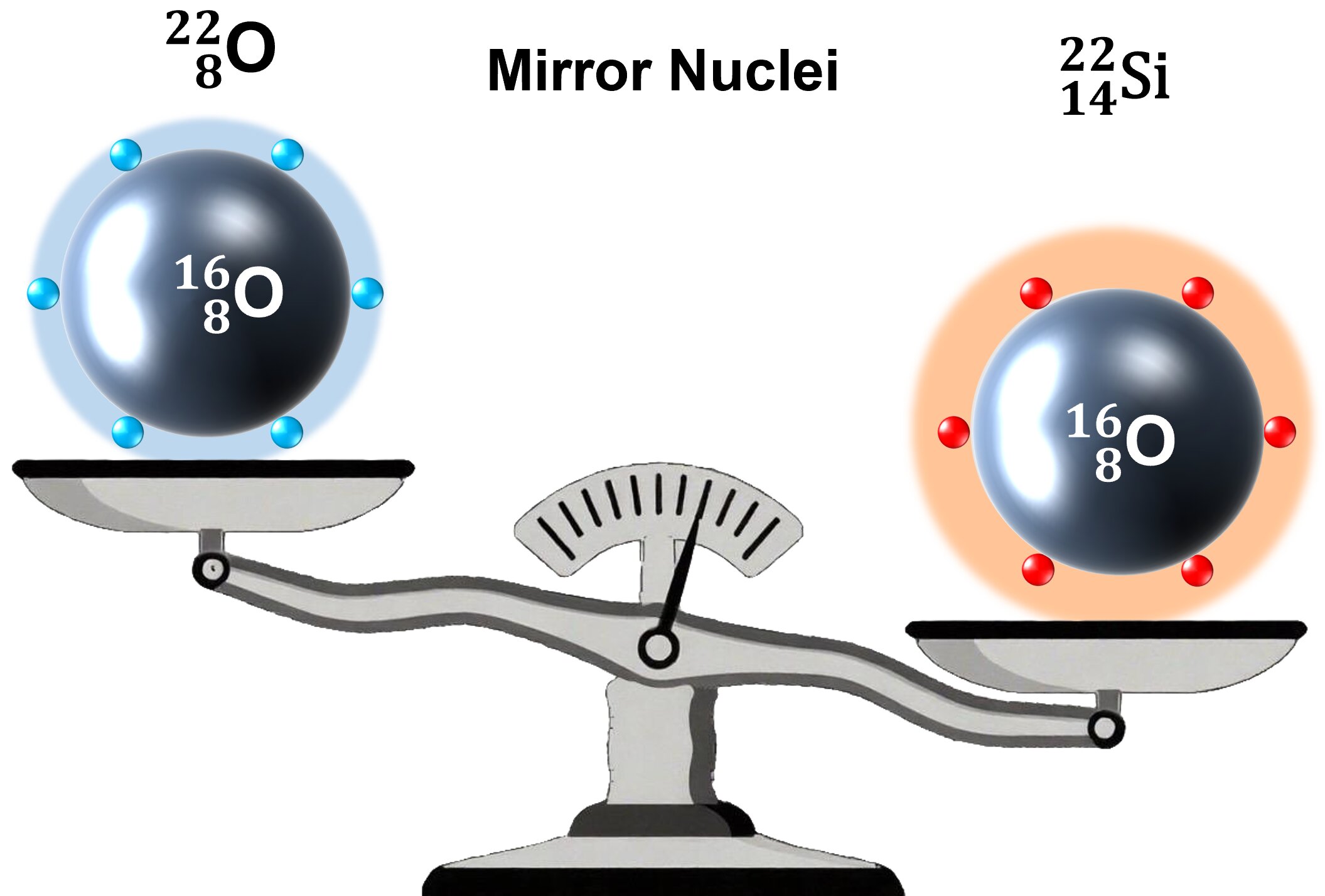
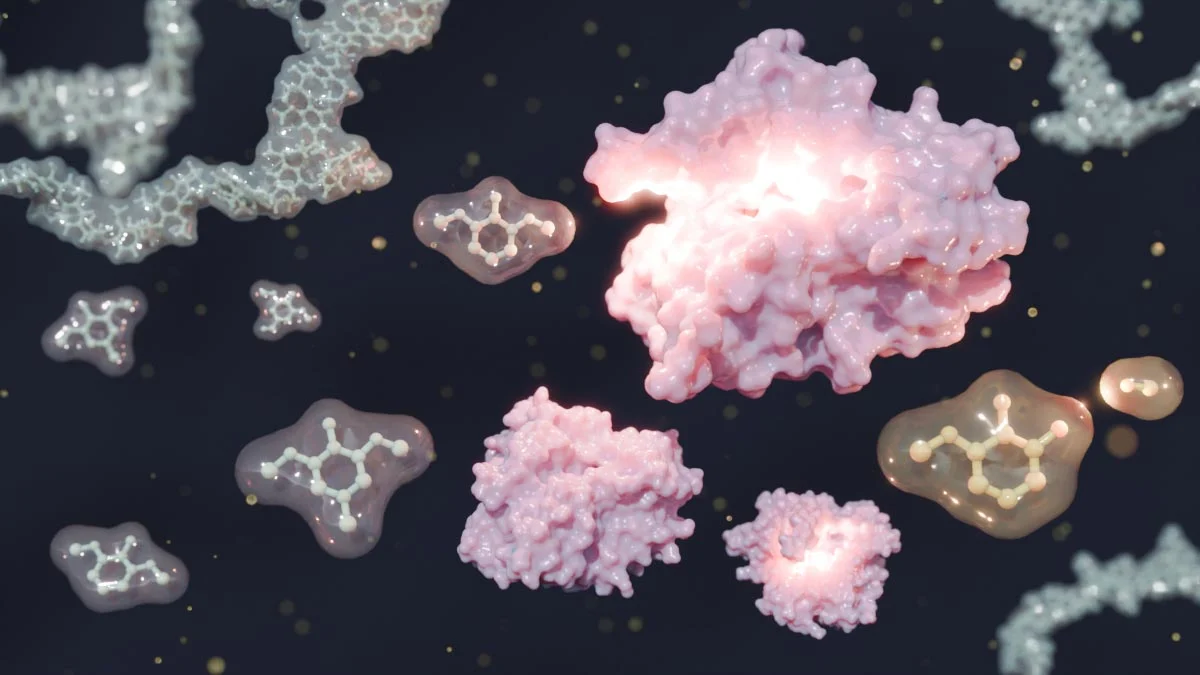
Structural schematic diagram of silicon-22 and its mirror nucleus oxygen-22. Through mass measurements of silicon-22 combined with theoretical calculations, researchers have revealed that silicon-22 possesses a double-magic structure similar to that of oxygen-22, while also exhibiting slight symmetry breaking with a more extended proton spatial distribution. Credit: IMP
Did scientists just crack a hidden code inside atomic nuclei?
It may sound like science fiction, but researchers at the Institute of Modern Physics (IMP), Chinese Academy of Sciences have revealed something extraordinary. By making ultra-precise mass measurements, they found that silicon‑22 (Si‑22), a rare isotope, has a special property. It shows evidence of a new “magic number” at proton count 14. This discovery changes how we understand nuclear stability.
Who Made the Discovery?
In nuclear science, certain numbers of protons or neutrons create stable configurations called magic numbers. Known examples include 2, 8, 20, and 28. These numbers complete nuclear shells, much like full electron shells in atoms. But Si‑22 lies in a difficult region to study—it exists on the edge of stability. To explore it, scientists used an advanced mass spectrometer at IMP’s Lanzhou facility.
What Did They Learn?
- Stability Confirmed: Si‑22 is stable enough to measure. It has a positive two-proton separation energy, meaning it does not immediately decay.
- Proton Shell Closure: When compared with its mirror nucleus, oxygen‑22, Si‑22 showed signs of a complete proton shell at Z = 14.
- Structural Differences: Using advanced nuclear models, the team found that protons in Si‑22 are slightly more spread out than neutrons in its mirror. This shows a small but important difference in structure.
- Rare Double-Magic Status: Because both protons and neutrons appear to form closed shells, Si‑22 may qualify as a double-magic nucleus—a rare and important category in nuclear physics.
Why It Matters
Magic numbers are like the load-bearing beams of an atom. They make certain nuclei more stable than others. Finding a new proton magic number means physicists must update their nuclear models. It also helps explain how matter behaves in extreme conditions, like inside stars or during supernova explosions.
In addition, this discovery shows the power of modern tools. With high-precision instruments, scientists can now measure tiny and unstable isotopes that were once beyond our reach.
What Comes Next?
Now that scientists have confirmed Z = 14 as magic, they will search for other rare isotopes with similar properties.
They also aim to improve nuclear models to better predict unusual behaviors in mirror nuclei.
Read the Original Study
Physical Review Letters published the full study on July 2, 2025.
DOI: 10.1103/PhysRevLett.134.012701
Explore More Discoveries
If you’re curious about atomic science, read how neutrino mass experiments are narrowing the mystery, or explore how the Higgs mechanism was confirmed through W-boson scattering.
Join the Discussion
Could more magic numbers be hidden in nature? What might they reveal about the building blocks of matter? Leave your thoughts in the comments and visit our nuclear science section for more stories.